Hydrogen peroxide is an effective oxidation reagent, which is used in a multitude of applications in chemical industry, in the pulp and paper industry and as bleaching agent. As water is the sole byproduct of the oxidation reaction, Hydrogen peroxide is considered environmentally benign. Today’s technology for the manufacturing of Hydrogen peroxide is the antraquinone process. This process encompasses several steps and can’t be downscaled economically. The direct synthesis of hydrogen peroxide from the elements was published already in the beginning of the 20th century. However, mainly due to safety reasons there is no commercial application as of now. Furthermore the consecutive reaction of hydrogen peroxide to water has to be effectively suppressed. Research in catalysts is ongoing here.
At the Institute of Micro process engineering (IMVT) a novel micro membrane reactor has been developed to overcome safety and reactions technological problems.
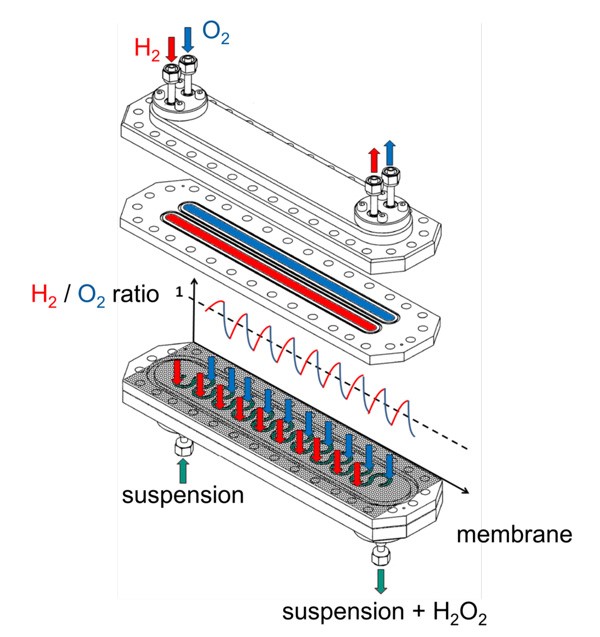
In our concept the reaction takes place in a meandering micro channel. The reaction medium is a liquid solvent, in which the catalyst is suspended. Oxygen and hydrogen are brought in via a membrane and react at the catalyst to form Hydrogen peroxide. The catalyst can be applied as suspension as packed bed or as wall coating.To increase yield the reactants are resaturated alternately.
Since the reaction takes place in liquid phase no explosive atmosphere is formed. The saturation of the surface layer of the liquid is effective in micro structure since the diffusion length is not small in reference to the channel depth. The suspended catalyst effects a pseudo-homogenous reaction throughout the reaction volume.
Publications:
Pashkova, A.; Dittmeyer, R.: Carbon dioxide as an alternative solvent for the direct synthesis of hydrogen peroxide: A review of recent activitie . 2015. Catalysis Today, 248, 128-137. doi:10.1016/j.cattod.2014.03.012
Dittmeyer, R.; Grunwaldt, J.-D.; Pashkova, A. A review of catalyst performance and novel reaction engineering concepts in direct synthesis of hydrogen peroxide. 2015. Catalysis Today, 248, 149-159. doi:10.1016/j.cattod.2014.03.055
Selinsek, M.; Pashkova, A.; Dittmeyer, R. Numerical analysis of mass transport effects on the performance of a tubular catalytic membrane contactor for direct synthesis of hydrogen peroxide. 2015. Catalysis Today, 248, 101-107. doi:10.1016/j.cattod.2014.05.048
Selinsek, M.; Bohrer, M.; Vankayala, B. K.; Haas-Santo, K.; Kraut, M.; Dittmeyer, R. Towards a new membrane micro reactor system for direct synthesis of hydrogen peroxide. 2016. Catalysis today, 268, 85-94. doi:10.1016/j.cattod.2016.02.003
B. J. Deschner, M. Selinsek, D. E. Doronkin, T. L. Sheppard, J.-D. Grunwaldt, R. Dittmeyer
Direct synthesis of H2O2 in continuous flow: an operando XAS study on palladium catalysts (oral)
13th European Congress on Catalysis, EUROPACAT 2017, 27th–31st August 2017, Florence, Italy
B. J. Deschner, M. Kraut, M. Selinsek, R. Dittmeyer
Untersuchungen zur Direktsynthese von Wasserstoffperoxid in einem suspensionsdurchströmten Membran-mikroreaktor (poster)
Jahrestreffen der ProcessNet-Fachgruppe Mikroverfahrenstechnik, 9th–10th March 2017, Frankfurt, Germany
M. Selinsek, B. J. Deschner, D. E. Doronkin, T. L. Sheppard, J.-D.Grunwaldt, R. Dittmeyer
Revealing the Structure and Mechanism of Palladium during Direct Synthesis of Hydrogen Peroxide in Continuous Flow Using Operando Spectroscopy
ACS Catalysis 2018, 8, 2546-2557, DOI: 10.1021/acscatal.7b03514