Hydrogen as energy carrier has a fundamental disadvantage: The missing distribution system and the relative low energy density of hydrogen in relation to the storage volume. The decentralised and mobile reforming of hydrocarbons could be an alternative way towards the hydrogen economy and the long-term usage of biofuels. The development of PEM fuel cells is far advanced, so that it is applied very often. However, the removal of carbon monoxide from the reformate gas mixture is mandatory. The separation of hydrogen by a membrane, the selective methanation of CO in presence of CO2 and the the preferential oxidation of CO are possibilities to reduce CO. A further possibility is the water gas shift reaction:
Because the water gas shift reaction is an exothermal balance reaction, adjusting the temperature profile in the microreactor is a possibility to minimize the dimensions of a reactor by running a maximum reaction rate in dependence of the CO-conversion and thermodynamic equilibrium. The optimization of the temperature control and the preparation of special catalysts is the focus at IMVT. The work is related to the SO2 oxidation
and the high pressure water gas shift reaction in biomass gasification
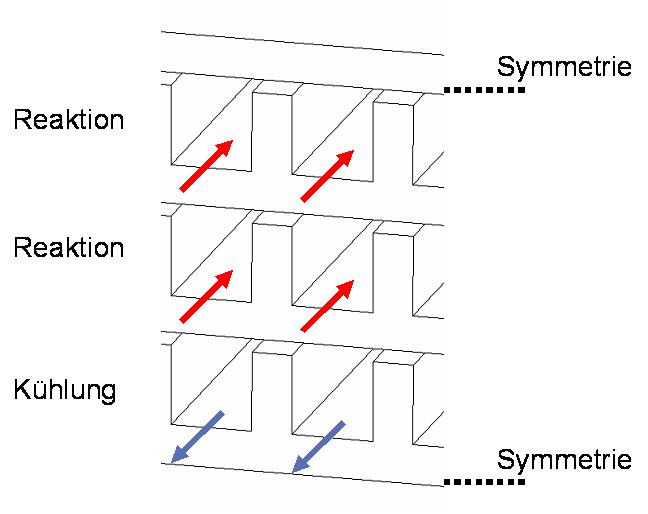 Schematic structure of microreactors with counter-flow cooling for the water gas shift reaction and SO2-oxidation according to modelling studies.
Schematic structure of microreactors with counter-flow cooling for the water gas shift reaction and SO2-oxidation according to modelling studies.