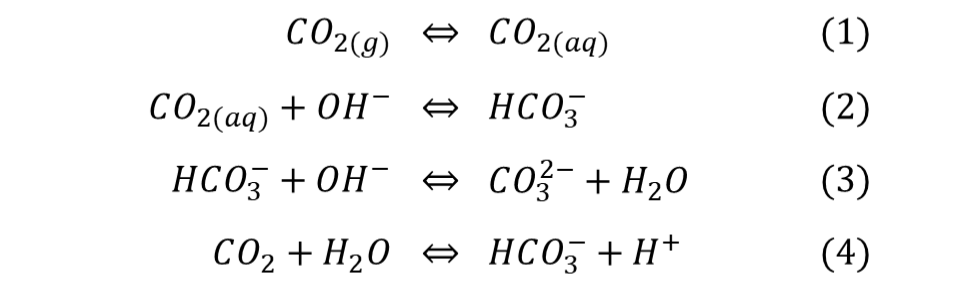Investigation of the influence of transport processes on chemical reactions in bubble flows using space-resolved in-situ analytics and simultaneous characterization of bubble dynamics in real-time
- contact:
- funding: DFG SPP 1740: The influence of Local Transport Processes on Chemical Reactions in Bubble Flows
- Partner:
University of Stuttgart, Institute for Parallel and Distributed Systems (IPVS), Stuttgart, Germany
- startdate: 2014
- enddate: 2017
By developing new experimental techniques, important contributions to an improved understanding of the interaction between hydrodynamics and chemical reactions in bubble flows shall be achieved. In this (particular) case the experimental methods are based on two systems.
Project abstract
By developing new experimental techniques, important contributions to an improved understanding of the interaction between hydrodynamics and chemical reactions in bubble flows shall be achieved. In this (particular) case the experimental methods are based on a novel Real-Time Raman-Process-Analysis-System that allows for a spatially and temporally-resolved in-situ analysis of the liquid phase in the vicinity of the simultaneously observed bubble. This system is an extension of the Real-Time-Process-Analysis-System, which was implemented by the applicant Professor Simon in another DFG-Priority-Program, and the in-situ Raman spectrometer established by Dr. Rinke, which shall be further developed here and integrated with the Real-Time-Process-Analysis-System.
With this system the kinetics of chemical reactions in relation to the bubble dynamics will be investigated in detail. In correlation with the observation of bubbles and bubble shapes, the spatially-resolved analysis yields the relevant information about the substance input. In this regard, an extensive automated analysis of bubble shapes will be conducted to measure a large number of bubbles and thereby obtain statistically meaningful results. In this project, bubbly flows, bubbles immobilized by counter-flow with and without the influence of turbulence (grid installations) as well as Taylor-flows within microchannels are to be investigated.
In order to determine the chemical composition, the Real-Time-Process-Analysis will be used to determine the local substance concentration for each laser pulse at one or more measurement points within a bubble flow at a defined point in time. To achieve this task, a complete Raman spectrum is to be recorded so that chemical reactions with complex Raman spectra can be evaluated and the Real-Time-Raman-Process-Analysis-System is used and applied in a flexible and universal way. Simultaneously with the recording of the Raman spectra, the rising bubbles, bubbles swarms and the surrounding flow field are monitored with high frame rates such as 1000 frames/s, to monitor/track the trajectory and dynamics of the observed rising bubble, e.g. to determine the measurement point or the measurement points for the Raman spectroscopy with respect to the position of the bubble in real-time. This is done by the Real-Time-Raman-Process-Analysis-System by determining the position of the bubble of each image sequence in real-time using an integrated image analysis mechanism. With respect to the experimental arrangement, the optical axis of the Real-Time-Raman-Process-Analysis-System is perpendicular to the flow direction of the bubbles in the bubble channel, so that the system can be integrated laterally in the test array and a 2D top view of the bubbles can be recorded and analyzed in real-time.
First application is the CO2 absorption. In this case, CO2 and caustic soda move as a Taylor-flow in a glass capillary with 2 mm x 2 mm inner cross section.
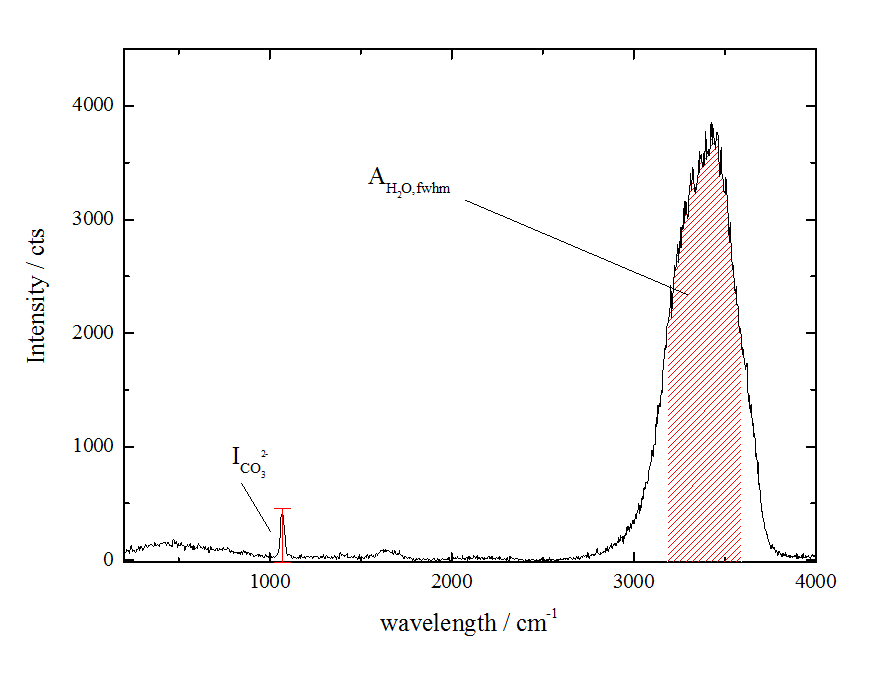
To measure the concentration of CO32-, its Raman line at 1080 cm-1 was used. For normalization purpose the Raman band of H2O was used. The picture shows a Raman spectrum, which was recorded with one laser pulse, only. With such spectra the concentration profiles between gas bubbles can be determined. The limit of detection for CO32- amounts to 0.07 mol/.