Simulation of a heterogeneous gas-phase reaction.
Sulphuric acid is one of the most used chemicals in the world. The precursor of sulphuric acid is sulphur trioxide (SO3), which is gained by the oxidation of sulphur dioxide with air via platinum or V2O5 a catalyst in an exothermic process. The reaction in a microstructured device offers great advantages and is the subject of intensive investigations on the IMVT. Therefore a microstructured reactor was developed by which it should be possible to oxidize SO2 to SO3 in a one-step process with platinum as catalyst. The advantage of the reaction in microstructured reactor is that due to the one-step process no stepwise cooling should be necessary. One aspect is the choice of material due to the high temperatures which are necessary for the catalytic oxidation of SO2. Only metallic materials meet also the additional demands of high heat transfer properties, corrosive stability and maintain the system stability up to 550°C. For this reason titanium as material has been chosen.
For the experiments several sulphur dioxide/oxygen mixtures flow through the platinum coated micro channels and are catalytically converted. Experiments have been performed which are different in composition sulphur dioxide/oxygen, hydrodynamic residence time and reaction temperature. Concerning the pressure drop of the microchannels, every channel is pressurized with the same mass flow. And due to the stability of the temperature distribution the microstructured reactor can be regarded as isotherm. So it can be assumed that every microchannel behaves essentially alike and therefore it is sufficient to simulate only one single channel.
The conditions for experiment/simulation are shown in the following table:
| Experiment | T,°K | Vol.flow_rate, ml/min STP | mass_fraction YSO2, - | mass_fraction YO2, - | mass_fraction YN2, - |
|---|---|---|---|---|---|
| T9 | 673, 748, 803 | 833 | 0,28 | 0,28 | 0,44 |
| T7 | 673, 748, 793 | 840 | 0,35 | 0,35 | 0,3 |
| T5 | 673, 748, 808 | 483 | 0,22 | 0,23 | 0,55 |
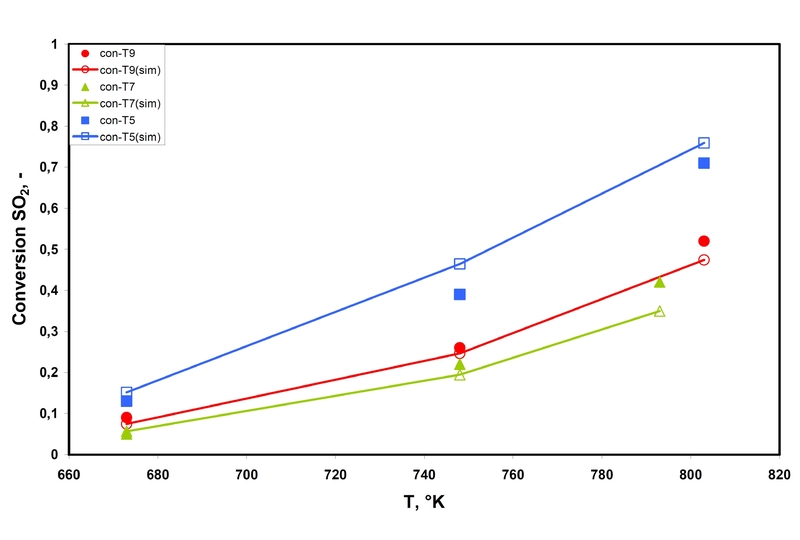 Figure 1: Conversion of SO2 as a function of temperature for the experiments T9, T7 and T5. Filled symbols describe the experimental values; lines with open symbols stand for the simulated values.
Figure 1: Conversion of SO2 as a function of temperature for the experiments T9, T7 and T5. Filled symbols describe the experimental values; lines with open symbols stand for the simulated values.In Figure 1 the conversion of SO2 as function of the temperature for the experiments according to the above table are shown. For all experiments a good agreement between the experimentally determined values and the simulation results are found. The yield is equivalent to the conversion due to the selectivity of 1. The conversion of SO2 continuously increases nonlinear with temperature.
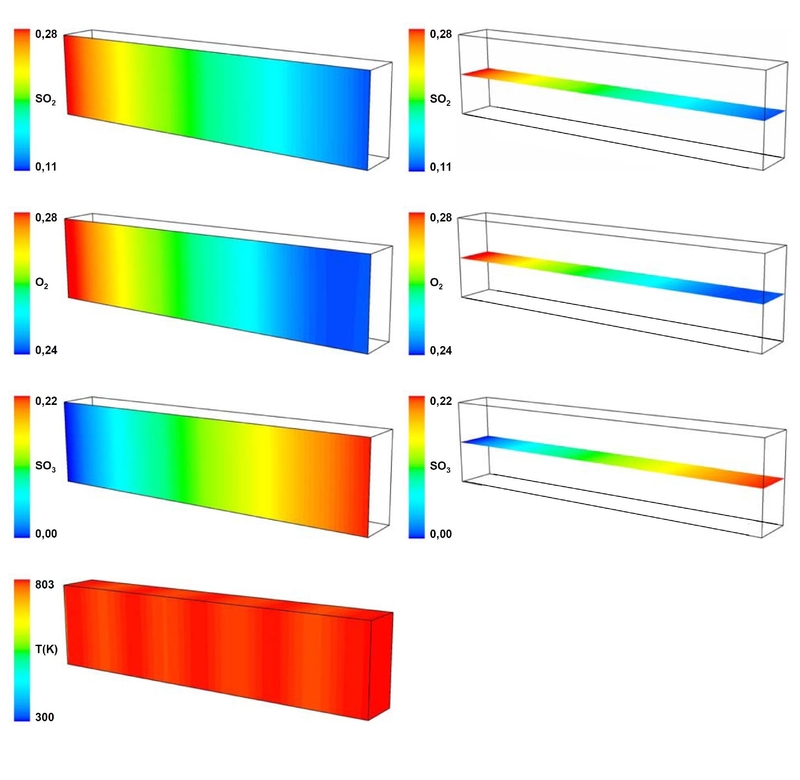 Figure 2: Contour plots of the mass fractions of SO2, O2 and SO3 and temperature as function of the channel length for experiment T9 for 673K; different length scales are used in radial and axial direction. Flow direction is from left to right.
Figure 2: Contour plots of the mass fractions of SO2, O2 and SO3 and temperature as function of the channel length for experiment T9 for 673K; different length scales are used in radial and axial direction. Flow direction is from left to right.In Figure 2 contour plots of species mass fractions and temperature are shown exemplarily for the experiment T9 for the temperature of 803K. These plots represent 2 faces in the xz-plane as well as in the yz-plane of the simulated half of the microchannel orientated along the horizontal and vertical symmetry plane. The tempera-ture is shown on the outer surfaces of the channel.
The profiles show, that the reagents are converted over the whole length of the micro channel. The mass fraction of the product reaches its maximum value at the outlet of the micro channel. In the radial range no gradients of the mass fractions can be observed. The temperature profile exhibits nearly isothermal conditions in axial as well as in radial directions. Temperatures near the inlet fall below the temperature levels of 803 K, respectively. This relatively small region is not visible in the contour plot. On the wall surface of the channel slight differences in the colour can be recognized. These colour differences give a hint of temperature fluctuations in axial direction. The temperature losses are caused by heat transfer to the environment. In the simulation with ANSYS FLUENT 12.1 the oxidation of SO2 in a micro channel with Pt as catalyst based on a self-developed multi-step mechanism as well as the thermal processes are described in good agreement with the experimental results.
